UTI vaccines like Uromune, Uro-vaxom, and Solco-Urovac are available in the UK and we’re gathering information about their availability elsewhere. At this stage, we can confirm that Uromune is available in the US via a special ordering mechanism. If you are interested in learning more about this option, you can register your interest here for more information. (UPDATE: As of July 2023, shipments of Uromune to the US have been paused. We will provide an update when shipments resume).
The vaccines mentioned above can be helpful for people who have access and are looking to prevent one of the few bacteria that the vaccines cover. But for people who do not have access or need to cover different bacterial strains, these UTI vaccines fall short.
With these limitations in mind, a Duke University team has been working to bring a UTI vaccine to the US. Additionally, their vaccine can target a significantly larger number of bacterial strains.
In this series of interviews, we are thrilled to introduce Dr. Soman Abraham, Grace Kerby Professor of Pathology at Duke University. Dr. Abraham has been contributing to the understanding of UTIs for nearly thirty years.
Dr. Abraham has been working with the team at Duke University to study the behaviors of specialized cells in the bladder, in an effort to develop a more effective UTI vaccine. Through these efforts, the team has managed to create a UTI vaccine that has not only been shown to prevent UTI, but treat active infections as well.
Dr. Abraham shared these fascinating insights with us and filled us in on how patients can become involved to help launch clinical trials around the vaccine more quickly. You can watch our three-part interview or read a quick summary below.
Jump To Section:
- The Race Between Bacterial Eradication and Bladder Tissue Repair >>>>
- A Vaccine That is More Than UTI Prevention >>>>
- How Do Side Effects and Uses Compare to Other UTI Vaccines? >>>>
- How You Can Get Involved in UTI Vaccine Development >>>>
The Race Between Bacterial Eradication and Bladder Tissue Repair
In discussion of the basic mechanics of UTIs and the immune system, we learn an essential component as to why antibiotics and vaccines are often unsuccessful. To begin, Dr. Abraham explains that UTI-causing bacteria go through a process that ends with them settling in the bladder lining. The bacteria, or other organisms, enter the bladder through the urethra, multiply in the urine, bind to the surface of the bladder lining, and finally, cause infection within the lining itself.
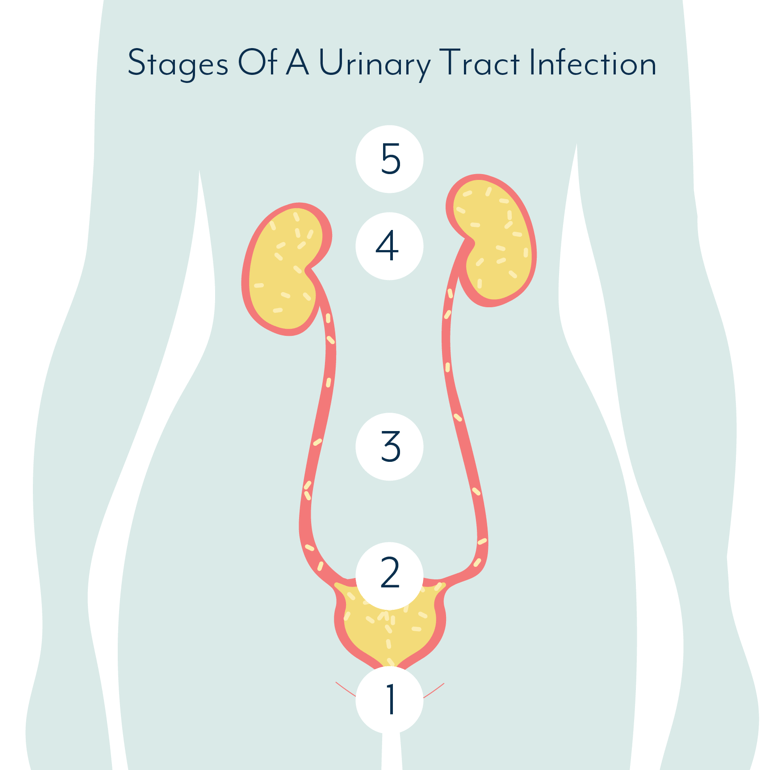
It’s important to note that the bacteria can go on multiplying while in the urinary tissues. This is where we encounter the trickiness of preventing recurrent UTIs.
The immune system’s response is to shed the bladder lining entirely, taking bacteria with it. However, there is a problem with this. The underlying tissue is now exposed to urine and the ammonia, salts, etc, therein, which can cause a great deal of pain.
Continuing on heroically, the immune system then sends in two types of T-cells to kill off any remaining bacteria (TH1 T-cells) and repair the bladder lining (TH2 T-cells). But in order to save the body from further pain, the repair bit tends to occur faster than the bacteria-killing bit. This can mean that some residual bacteria are left to hide, safe and snug, within the urinary tissues. Multiplying within these tissues, the result is often recurrent UTIs.
Moreover, this same cycle is exacerbated with each subsequent UTI. With more exposure, the body tries harder and harder to repair itself while neglecting to get rid of the ever-multiplying supply of bacteria.
Having found a very effective means of shelter, antibiotics are often unable to reach these embedded bacteria. This explains why so many patients find antibiotics ineffective.
A UTI Vaccine Shift Differing from Uro-Vaxom and Uromune
Vaccines, such as Uromune, Uro-Vaxom, and Solco-Urovac, seem to have similar trouble to antibiotics in accessing these embedded bacteria. The existing vaccines all have slightly differing methods of administration, with most being taken orally or sublingually (under the tongue).
While administration methods vary, all aim to protect against E. coli by stimulating the immune system to produce IgG antibodies. These antibodies are created in the mucosa of the body. By exposing the mucosa in the mouth to the vaccine, the bladder mucosa may also develop antibodies.
Up until now, the role of T-cells in the bladder that we mentioned previously has been overlooked. Dr. Abraham has taken the data his team gathered around T-cells in bladder research, as well as data around the current UTI vaccines, to work on developing a vaccine with a special focus on the importance of attracting TH1 T-cells specifically, the kind that kill bacteria.
As more THI T-cells populate the bladder, bacteria will, theoretically, be given no opportunity to hide beneath the repaired lining.
Another shift Dr. Abraham is making with this vaccine as opposed to Uro-vaxom and others is the administration method. Instead of a sublingual, oral, injection, or vaginal suppository vaccine, the vaccine will be administered through a urinary catheter. A small dose (three in total) will be instilled, and the patient will urinate normally a couple hours later.
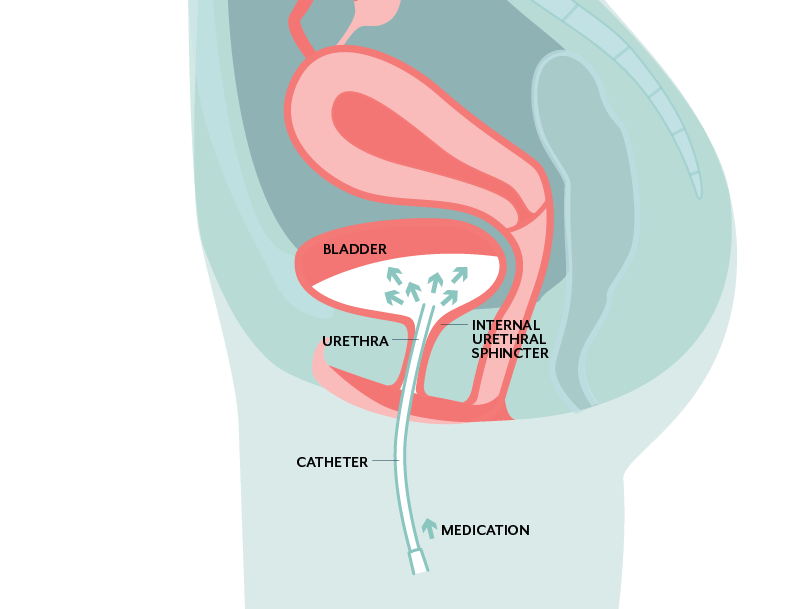
As more TH1 T-cells move to the bladder, the bladder may have the opportunity to shed the infected epithelial cells before the bladder tissue begins regenerating.
A Vaccine That is More Than UTI Prevention
While Dr. Abraham’s vaccine is still subject to further testing and observation, thus far, it has shown the potential to clear all bacteria in mice bladders within the last two weeks of the vaccination process.
It’s likely that once it reaches its full effectiveness, the vaccine will be able to be used as both a treatment and method of prevention. The TH1 T-cells will strategically locate themselves within the bladder lining, and be prepared to activate if another infection emerges.
As far as the duration of protection, Dr. Abraham believes that the evidence they have collected to date in mice studies demonstrates that the protection will be longer lasting than that of the other antibody-producing vaccines, such as Uro-vaxom.
While this is the expectation, it should be noted that long-term studies have not yet been completed. Booster instillations may be necessary to enhance the duration of effectiveness.
How the Duke University UTI Vaccine Ingredients Differs from Uro-vaxom
In its basic form, the vaccine contains two active components. The first being FimH proteins, the protein on the outside of E. coli. The second component is CPG single-stranded dna molecules, which are responsible for producing an antibody response. These ingredients have a number of benefits:
- Minimal risk of side effects – the direct application into the bladder means the likelihood of it affecting the microbiome of the gut, skin, or other organs is very low. Moreover FimH and CPG have not been shown to be toxic when otherwise tested individually. It should be noted that they have not yet been tested together in humans.
- Direct and customizable treatment – the vaccine is designed to address issues of E. coli and the 100+ gram-negative species of bacteria within the enterobacteriaceae family. However, its simple structure allows flexibility to customize it in order to address other potential variants in infection. With the proper testing completed, it should even be able to address fungal components.
- 0 interaction with antibiotics – the mechanism of action has no relation to antibiotics, so there should be no concern over having both in the system at once. While antibiotics are targeting free-floating bacteria, the vaccine can decrease persistence of the infection in the tissues.
- 0 heavy metals – the lack of heavy metals should relieve patients’ concern for related toxicity lingering in the body.
One possible downside to the T-cell function of the vaccine is that it’s unknown whether or not T-cells can target bacteria inside biofilms. When it comes to intracellular bacteria, the vaccine from the Duke University team shows promise as it differs from the inability of Uromune, Uro-vaxom and the like to reach embedded bacteria.
How Do Side Effects and Uses Compare to Uro-Vaxom?
Continuing on with the discussion around side effects and the benefits of the Duke University team’s vaccine, Dr. Abraham again stresses that the primary components of the vaccine are innocuous. Neither FimH nor CPG are individually toxic, and he does not believe the combination will result in a negative response.
Of course, safety studies will still be completed to ensure this expectation is accurate.
Dr. Abraham goes so far as to state his confidence that even pregnant women are unlikely to have any cause for anxiety, due to the vaccine being used directly in the bladder.
Additionally, based on current evidence, the risk of the UTI vaccine interacting with other medications, including existing vaccines like Uro-vaxom, appears to be low. In fact, there is a chance that Dr. Abraham’s vaccine may boost the effectiveness of something like Uro-vaxom.
For people who are post-menopausal, have a spinal injury or interstitial cystitis (IC), as long as the immune system is functioning, the vaccine will be able to bring T-cells to the bladder and decrease UTI risk. Dr. Abraham points out that if a person’s IC has a bacterial etiology, the vaccine will of course be able to both prevent and treat UTIs.
How You Can Get Involved in UTI Vaccine Development
After reading about Dr. Abraham’s UTI vaccine development in a press release, patients in our UTI community reached out, eager to become involved. One of the first steps in producing this vaccine so it’s widely available is completing safety and efficacy studies.
Studies such as this have a series of steps. As part of the study development, funding needs to be established and markers to determine success of the vaccine need to be established before trials can begin.
However, patients don’t need to wait for clinical trials to begin in order to get involved. If you’re interested, you can be a voice in expressing the need for a vaccine that performs in this way. Unfortunately, many funding agencies are composed of people who don’t recognize the gravity of UTI, as it’s primarily a women’s health issue. This is one of the reasons it’s so important for patients to share their perspective when it comes to research.
We may share an opportunity for you to become involved in this way. Make sure you’ve subscribed to receive our emails to be notified of details around this possible campaign.
Read Dominique’s story and her experience with the Uromune vaccine.
Acknowledgements
We want to thank Dr. Soman Abraham for taking the time to answer our questions about the new UTI vaccine. Although other UTI vaccines already exist, most are not readily available outside of the UK with the exception of Uromune. If you are interested in learning more about Uromune and how to order it, you can register your interest here for more information. Please note that as of July 2023, shipments of Uromune to the US have been paused. We hope to have an update on when shipments to the US will be resumed soon.
This new vaccine that Dr. Abraham is working on would not only be made available in the US, but the additional T-cell component brings new potential for chronic and recurrent UTI patients.
Subscribe to Live UTI Free on YouTube to watch more expert interviews and to be notified when new videos are released.
To get answers to commonly asked questions about chronic and recurrent UTI, visit our FAQ page. Share your questions in the comments below, or reach out to our team directly.
Ask Questions. Tell Stories!
Comments
Any updates on vaccines/vaccine availability in the US?
Hi Shelby, I’ve sent some information via email. Best wishes, Issy
Hi, are there any updates on this? Have the human clinical trials started? I emailed the Duke team a couple of months ago but no response. Thank you!
Hi, I’ve sent an email with some more information to you. Best wishes, Issy
My Mom is a quadriplegic that has lived with reoccurring UTI’s for over a decade. She is 75 years old and she needs this vaccine.
Hi John, if you have any specific questions about this vaccine, please get in touch via the online contact form: https://staging.liveutifree.com/contact/
Best wishes,
Issy
Hello, my wife has a urostomy because of past renal TB and has struggled with recurrent UTIs. We have used urovaxon and uromune, but Klebsella is a frequent issue for here, as well as e coli.
Could we get an update on the vaccine? how can I know more about it.
Hi Daniel, we’re also waiting for an update on this vaccine trial, and we hope to hear something more soon. Melissa
I am a quadriplegic living in AZ and have been suffering with chronic UTIs for 40+ yrs. I am very excited about the possibilities of Uromune or other vaccine. Is there anything I can d to get involved? Thx!
Please keep me abreast of any updates on vaccines for recurrent utis.
Hi Donna, we sent you an email and you’re welcome to join our mailing list for updates. Melissa
Hi Rick, I have sent you an email. Let me know if you have any questions, Molly.
I am too desperate to know if the vaccine is out. I live in UK. I m willing to contribute financially too for this vaccine
Hi Khem, I have emailed you. I hope it helps, Molly.
Thank you for this article. I have been following this vaccine development and find it so hopeful. I see this was updated January 1, 2024. Could you tell me what the new update is? Is there even a loose timeline for trials? And is there anything holding this up? I wonder about funding. Thank you so much!
Hi Hannah, I have emailed you, Molly.
So if you supress t cells would you have a better chamce of clearing the infection? Im asking because I was on cyclosporine and when I was I didnt get many uti’s and its an immunosuppresant. I also have Sjogrens Syndrome
Hi Michelle, that’s an interesting question in light of Dr. Abraham’s theory. We don’t have the answer to that at this stage, though. I will keep an eye out for research on this. Melissa
I’m so tired of UTI’s. 74, and live in NC. Please send information!
Hi Francine, I just emailed you more information. I hope it helps, Melissa
Hi Melissa, is it possible for someone with abnormal kidney function to get the vaccine? if yes will it make a difference and maybe even improve the kidney function if at all?
Hi William, I have emailed you. I hope it helps, Molly.
How old do men have to be to get the UTI vaccine?
Hi William, we sent you an email with more information. I hope it helps, Melissa
Where can I get a UTI vaccine? I am willing to go to another country
Hi Julie, I just emailed you with more information. For anyone else interested, you can either click on the link to register your interest (in the article above) or message us directly to let us know where you are based and that you are interested in UTI vaccines. Thanks! Melissa
Are there trials going on now? I wish I could be a part of this.
Hi Jessica, not yet, but we will make an announcement when we hear any updates. Melissa
I really want to be a candidate in the UTI vaccine being developed at Duke, how can I volunteer?
Hi Gloria, when the human trials begin, we will make an announcement to our community about how to participate. Melissa
Great! Please do. I am interested and so are several others in the embedded UTI Facebook group page.
That’s great to hear! We’ll definitely spread the word as soon as we know anything. Melissa